New Publication just released
The role of Lewis acidic vanadium centers in DME steam reforming over V-Ni catalysts
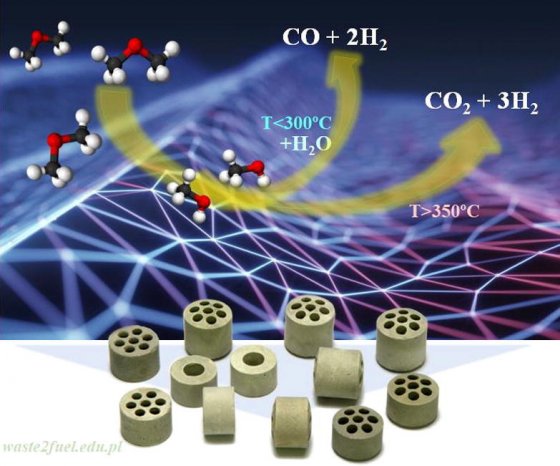
Dimethyl ether (DME) as a potential chemical storage of renewable energy was studied for decomposition and steam reforming (DME SR) to produce hydrogen-rich streams over V-Ni/Al2O3 catalysts. The extent of DME decomposition, DME hydrolysis, and subsequent methanol steam reforming was examined during this study, focusing on the redox and acid/base properties of catalyst surface species. The results show that the DME SR performance depended on the acid-redox character of the Ni-O-Ni/V-O-Ni species dispersed onto alumina. For S/C ratio close to 2.5, for relative V-rich catalysts, DME hydrolysis reaction over the Lewis acid sites was found a rate-determining step and then, metallic centers being responsible for H2 and CO2 production. For 3V-Ni/Al2O3 DME SR with low selectivity towards methane and direct H2 and CO2 production (H2/CO2 ratios close to 3), with near-complete DME conversion was obtained above 673 K.
Please see more at https://lnkd.in/eAuBmtd
The authors acknowledge the National Science Centre Poland for funding through project SONATA-2013/11/D/ST5/03007 ‘Second generation of biofuel and near future alternative fuels - development of stable, selective and highly active for steam reforming process’ awarded to I.S.P with connected contracting agreement 2012/10/11 for “DME process and material development’ given to C.H., M.L., L.A., and SONATA scholarship contracts no. 2014/12/15 and 2015/06/01 given to R.G.G. C.H., M.L., L.A. gratefully acknowledge the contract no. 20170000000000998 ‘Development and characterization of advanced catalytic materials’ trough Reintegration FNP 2016/1-5 ‘Waste into fuel - catalyst and process development for waste biomass valorization’. The UBA is acknowledged for TPR.